Magnetic Clusters



Research Projects
Polymerization
Current Funding
NSF CAREER,
CHE 0645414, (2007-2012)
UMSL Start-up (2008-2010)
Selected Publications
"Magnetic and Optical Bistability Driven by Thermally- and Photo-Induced Intramolecular Electron Transfer in a Molecular Cobalt-Iron Prussian Blue Analogue,” Li, D.; Clérac, R.; Roubeau, O.; Harté, E.; Mathonière, C.; Le Bris, R.; Holmes, S. M. J. Am. Chem. Soc. 2008, 130, 252-258.
"An S = 6 Cyano-bridged Octanuclear FeIII4NiII4Complex that Exhibits Slow Relaxation of the Magnetization," Li, D.; Parkin, S.; Wang, G.; Yee, G. T.; Clérac, R.; Wernsdorfer, E.; Holmes, S. M. J. Am. Chem. Soc. 2006, 128, 4214-4215.
"Syntheses, Structures, and Magnetic Charactierization of Dicyanometalate(II) Building Blocks: [NEt4][(Tp*)MII(CN)2] [MII = Cr, Co, Ni; Tp* = hydridotris(3,5-dimethylpyrazol-1-yl)borate]," Li, D.; Ruschman, C.; Parkin, S.; Clérac, R.; Holmes, S. M. Chem. Commun. 2006, 4036-4038.
“Early Metal Di- and Tricyanometalates: Useful Building Blocks for Constructing Magnetic Clusters,” Li, D.; Parkin, S.; Wang, G.; Yee, G. T.; Holmes, S. M. Inorg. Chem. 2006, 45, 2773-2775.
"Ancillary Ligand Functionalization of Cyanide-bridged S = 6 FeIII4NiII4 Complexes for Molecule-Based Electronics," Li, D.; Clérac, R.; Parkin, S.; Wang, G.; Yee, G. T.; Holmes, S. M. Inorg. Chem. 2006, 45, 7569-7571.
“Single Molecule Magnets Constructed from Cyanometalates: {[Tp*FeIII(CN)3MII(DMF)4]2[OTf]2}·2DMF (MII = Co, Ni)," Li, D.; Parkin, S.; Wang, G.; Yee, G. T.; Prosvirin, A. V.; Holmes, S. M. Inorg. Chem. 2005, 44, 4903-4905.
"An S = 2 Cyano-bridged Trinuclear FeIII2NiII Single-Molecule Magnet," Li, D.; Clérac, R.; Parkin, S.; Wang, G.; Yee, G. T.; Holmes, S. M. Inorg. Chem. 2006, 45, 5251-5253.
[1]
[2]
[3]
[4]
[5]
[6]
[7]
Introduction: Slow relaxation of the Magnetization in Clusters.
Single-molecule magnets (SMMs) are an interesting class of molecular clusters. These soluble, molecular, single domain superparamagnetic clusters exhibit high spin ground states, large and negative axial magnetic anisotropy (D < 0), low-symmetry molecular shapes (e.g. disk-shaped, butterfly), and high spin reversal barriers (S2D < 50 cm-1). The magnetic hysteresis and bistability exhibited by these clusters can theoretically be exploited for nanoscale memory applications but unfortunately, thermally activated magnetization reversal becomes energetically favorable at extremely low temperatures (ca. 4 K or less). Increasing this “blocking temperature” is of fundamental interest and technological importance (below).
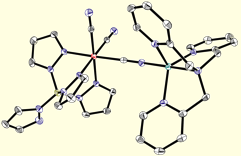
Di-, Tri-, and Tetranuclear Clusters.
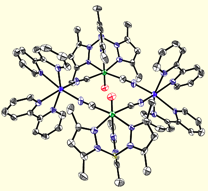
Given the robust nature of most transition metal cyanide linkages and the relative ease in which they assemble into well-defined structures, we we have found that facially-capped tris- and tetra(pyrazolyl)borate tricyanide complexes are useful for constructing magnetic clusters. The low-spin [(TpR,R)FeIII(CN)3]- (R = H, Me; S = 1/2) building blocks exhibit significant orbital contribution to the magnetic moment (g ~ 2.9) and upon treatment with a variety of divalent trifluoromethanesulfonate salts, afford a series of isostructural clusters that exhibit slow relaxation of the magnetization (above & below).
Octanuclear Clusters.
Utilizing ligands that are less sterically demanding, we have also prepared a variety of octanuclear molecular boxes of MIII4MII4 stoichiometry. A series of neutral and cationic cluster derivatives have been prepared (16+ to date) and magnetic measurements indicate that several exhibit slow relaxation of the magnetization. We have recently prepared several clusters and a few networks that also exhibit photoresponsive behavior.
Molecular shape anisotropy impacts the magnetic properties exhibited by structurally distorted clusters; distorted (twisted) clusters (rectangles and boxes) appear to be better SMMs than more symmetrical ones (rectangular or box-shaped). Magnetic and theoretical investigations are conducted in collaboration with Gordon T. Yee, Mike Whangbo, Rodolphe Clérac, and Kyungwha Park and are aimed at discovering the origins of this unusual and interesting behavior.
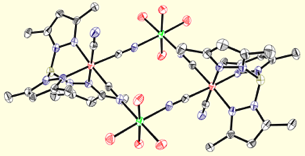
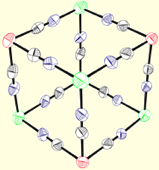
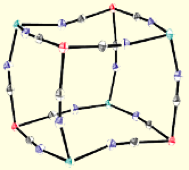
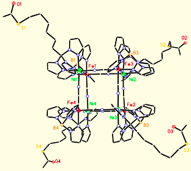
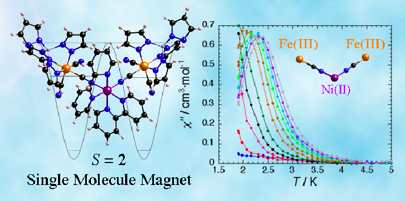
To construct cyanometalate clusters that exhibit slow relaxation of the magnetizaion, transition metal centers that possess single-ion anisotropy, either via spin state (large zero-field splitting parameters, D) or orbital anisotropy (large spin-orbit coupling parameters) must be present. Cyanometalates are excellent building blocks for constructing molecule-based clusters because cyanides generally form linear M-CN-M’ linkages, stabilize a variety of transition metal centers and oxidation states, and efficiently communicate spin density information. The sign and magnitude of the local exchange interactions can be controlled via substitution and predicted using simple orbital symmetry arguments. The cyanometalate ions used to construct these unusual complexes exhibit significant orbital anisotropy, which suggests that single-molecule magnetism is a general phenomenon.