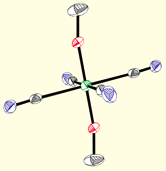
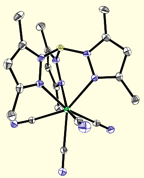
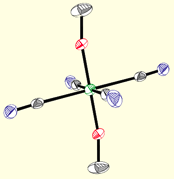
Current efforts investigate the preparation of new paramagnetic di-, tri-, and tetracyano complexes, many of which contain easily modified pyrazolylborate ligands. The robust mononuclear complexes (below) contain transition metal ions that exhibit angular momentum (spin-orbit) contributions to their magnetic moments, an important feature for engineering clusters that exhibit slow relaxation of the magnetization. Using a building block synthetic approach, aggregation of these precursors via M(CN)M' linkages into polynuclear derivatives allows for systematic tuning of the optical and magnetic properties of the resulting products and accurate structure-property relationships to be described.











Cyanide Complexes
Research Projects
Polymerization
Current Funding
NSF CAREER,
CHE 0645414, (2007-2012)
UMSL Start-up (2008-2010)










































































































































Selected Publications
"Syntheses, Structures, and Magnetic Charactierization of Dicyanometalate(II) Building Blocks: [NEt4][(Tp*)MII(CN)2] [MII = Cr, Co, Ni; Tp* = hydridotris(3,5-dimethylpyrazol-1-yl)borate]," Li, D.; Ruschman, C.; Parkin, S.; Clérac, R.; Holmes, S. M. Chem. Commun. 2006, 4036-4038.
“Early Metal Di- and Tricyanometalates: Useful Building Blocks for Constructing Magnetic Clusters,” Li, D.; Parkin, S.; Wang, G.; Yee, G. T.; Holmes, S. M. Inorg. Chem. 2006, 45, 2773-2775.
"Ancillary Ligand Functionalization of Cyanide-bridged S = 6 FeIII4NiII4 Complexes for Molecule-Based Electronics," Li, D.; Clérac, R.; Parkin, S.; Wang, G.; Yee, G. T.; Holmes, S. M. Inorg. Chem. 2006, 45, 7569-7571.
“Single Molecule Magnets Constructed from Cyanometalates: {[Tp*FeIII(CN)3MII(DMF)4]2[OTf]2}·2DMF (MII = Co, Ni)," Li, D.; Parkin, S.; Wang, G.; Yee, G. T.; Prosvirin, A. V.; Holmes, S. M. Inorg. Chem. 2005, 44, 4903-4905.
[1]
[2]
[3]
[4]
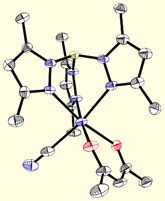










































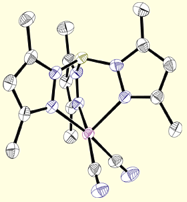
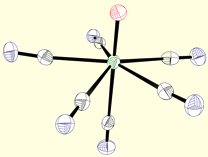
First-Row Building Blocks
Poly(pyrazolyl)borates are ideal ligands for directing the self-assembly of magnetic clusters: (1) These ligands are easily prepared and modified at each of their ten sunbstitutional positions, (2) Poly(pyrazolyl)borates stabilize multiple oxidation states for most transition metal centers (many are unknown for triazacyclononanes or triphos), (3) Pyrazolylborate complexes are known for most transition metals, (4) Allow for rapid tuning of the solubility, dimensionality, coordination preferences, and electronic properties of metal centers in a given structural archetype, and (5) Cyanometalate [fac-LmMn(CN)3](n-m-3) complexes and derived clusters appear to be stable with respect to linkage isomerism (a common problem in many systems). The structures of a few cyanometalate complexes are illustrated below.
Second- and Third-Row Building Blocks
We are currently investigating the preparation of second- and third-row poly(pyrazolyl)-borate tricyanide complexes in order to expand our studies concerning the role of single-ion anisotropy in molecule-based magnetic materials. Such building blocks are expected to afford stronger magnetic exchange couplings and molecular anisotropy, relative to first-row analogues, since increased pi backbonding and large orbital contributions (ca. 273–3000 cm-1) to the magnetic moment are often found for 4d and 5d ions. Inserting these late metal centers into clusters should further increase the spin reversal barrier energies and afford higher blocking temperatures, in comparison to known first-row cyanometalate-based analogues.