 |
Professor Beatty received her B.S. degree in chemistry from UMSL in 1989 and a Ph.D. from Washington University in St. Louis, in 1994. She held positions at Washington University, Kansas State University, the University of Notre Dame and Mississippi State University prior to joining the faculty at UMSL in 2008. |
beattya@umsl.edu |
Research Interests - (supported by NSF-Career award CHE 0645-758)
The goal of our research is to create new, useful solid or polymeric materials through use of organic, inorganic and supramolecular synthesis especially using techniques developed through crystal engineering. These new materials will provide a foundation for systematic structure-property studies, initially focusing on: electroactive polymers, catalysis, chiral separations, magnetic solids. Projects will utilize common synthetic routes and methods of characterization in solution and the solid state.
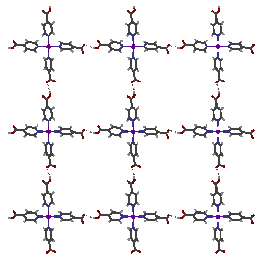
We have demonstrated that, despite the competing intermolecular forces that exist in solutions of coordination complexes, hydrogen-bonding substituents on ligands may be used to predictably assemble coordination complexes. We can control the solid-state assembly of inorganic/organic hybrid materials either by changing the metal ion (thus the preferred coordination geometry) or by synthesizing ligands with hydrogen bonding substituents. For example, the figure above shows that square planar Pt(II)(isonicotinic acid)2(isonicotinate)2 complexes are linked through carboxylic acid-carboxylate OH---O hydrogen bonds to form a square grid in the solid state.
Why crystalline solids? It is important to note that crystalline solids can, in some cases, be uniquely useful materials. By definition, single crystals are ordered, which means that structure-function (e.g. electronic or magnetic behavior) relationships can be determined by measuring the effect of systematic changes in the components of the crystal. In addition, channels or cavities organized in crystalline solids have equivalent environments, therefore the relative orientations of guest ions, molecules, or reactants are also constant, which is essential for: 1) uniform signaling in chemical sensors, 2) asymmetric catalysis, 3) stereochemically controlled solid state reactivity.
Selected Publications
″π-Choreography in aromatic ammonium formate solids,″ C. D. Oberle, D. G. Bequette, T. K. Brewer, T. R. Terry and A. M. Beatty, CrystEngComm. 2018, 20, 1899.
″Solid phase microextraction (SPME) combined with TGA as a technique for guest analysis in crystal engineering,″ M. J. Fischer and A. M. Beatty, CrystEngComm, 2014, 7313
″Synthesis of small pyridine building blocks," R. A. Bawa and A. M. Beatty, Rasayan J. Chem. 2012, 5, 496.
"Synthesis of some aminopicolinic acids," R. Bawa and A. M. Beatty, J. Chem. & Chem. Eng. 2012, 6, 372
"Stable Hydrogen-Bonded Coordination Network with Removable Guests", G. A. Hogan, N. P. Rath and A. M. Beatty, Crys. Growth Des. 2011, 11, 3740
"Exceptions to the rule: new hydrogen-bonded networks from an old reliable", O. Ugono, N. P. Rath and A. M. Beatty, CrystEngComm 2011, 13, 753
"2,2',5,5'-Tetrachlorobenzidine" O. Ugono, M. Douglas Jr, N. P. Rath and A. M. Beatty,Acta Crystallogr. E, 2010, 66, o2285.
"2,4,6-Triphenylaniline", O. Ugono, S. Cowin and A. M. Beatty, Acta Crystallogr. E,2010, 66, o1777.
"Synthesis and Control of Single Layer and Polar 2-D Layered Architectures in a Series of Organic Layered Solids", O. Ugono, N. P. Rath and A. M. Beatty, Cryst, Growth & Design 2009, 9, 4595.
"Guest Inclusion and Structural Dynamics in 2-D Hydrogen-Bonded Metal-Organic Frameworks" C-L. Chen and A. M. Beatty, J. Am. Chem. Soc. 2008, 130, 17222
"Reverse engineering: toward 0-D cadmium halide clusters" C. E. Costin-Hogan, C-L. Chen, E. Hughes, A. Pickett, R. Valencia, N. P. Rath, and A. M. Beaty, Cryst. Eng. Comm., 2008, 10, 1910-1915
"From Crystal Engineering to Cluster Engineering: How to Transform Cadmium Chloride from 2-D to 0-D," C-L. Chen and A. M. Beatty, Chem. Commun., 2007, 76..
"Metal-Containing Dicarboxylic Acids as Building Blocks for Lamellar Inorganic-Organic Hybrid Networks" A. M. Beatty, B. A. Helfrich, G. A. Hogan and B. A. Reed, Crys. Growth Des. 2006, 6, 122.
"Open-framework coordination complexes from hydrogen-bonded networks: toward host/guest complexes," A. M. Beatty, Coord. Chem. Rev. 2003, 246 (1-2): 131.
"Do polymorphic compounds make good cocrystallizing agents? A structural case study that demonstrates the importance of synthon flexibility," C. B. Aakeröy, A. M. Beatty, B. H. Helfrich and M. Nieuwenhuyzen, Cryst. Growth. Des. 2003, 3, 159